Speech impairments in Parkinson’s disease: the brain perspective.
Parkinson’s disease is a neurological disorder that progressively degrades the motor and cognitive abilities of affected patients. Although motor impairments are much more commonly studied in patients, their cognitive symptoms are also debilitating and deserve more focus.
Our new study published today in open access by npj Parkinson’s Disease clarifies the neurophysiological manifestations of speech impairments in Parkinson’s disease using advanced functional brain imaging.
We can easily imagine how debilitating and stressful it must be to patients with Parkinson’s disease to experience issues with speaking. This ability is essential to express needs and desires, and for maintaining social bonds. When social interactions are impaired because of challenged spoken communication, patients are exposed to further clinical decline.
Speech requires the subtle coordination of cognitive and motor processes, involving multiple regions of the brain. Although a primary source of anxiety and challenges in the patients experiences, speech impairments in Parkinson’s disease have been seldom studied. In particular, it was unclear whether they are primarily due to difficulties with the cognitive components of speech — such as planning articulatory movements — or with the actual movements of the jaw, mouth and larynx that are necessary to utter words.
In this new study, Alex Wiesman, PhD, NIH F32 post-doctoral fellow in our lab, has identified the origins of speech impairments in the brain of patients with Parkinson’s disease. To do this, we and our collaborators mapped brain activity in a large group of patients with magnetoencephalography (MEG) imaging. MEG identifies both the spatial origins of brain activity and how they change over time, millisecond by millisecond. We also recruited age-matched healthy participants in the study, to establish a benchmark comparison with the patients with PD. This new approach called the Spectral Deviation Index enabled us to derive for each patient a brain map of how and where their brain activity departs from healthy levels.
This approach implements a form of personalized brain mapping of dysfunctions in patients, which we had proposed in a review of the realm of possibilities with MEG mapping in Nature Neuroscience.
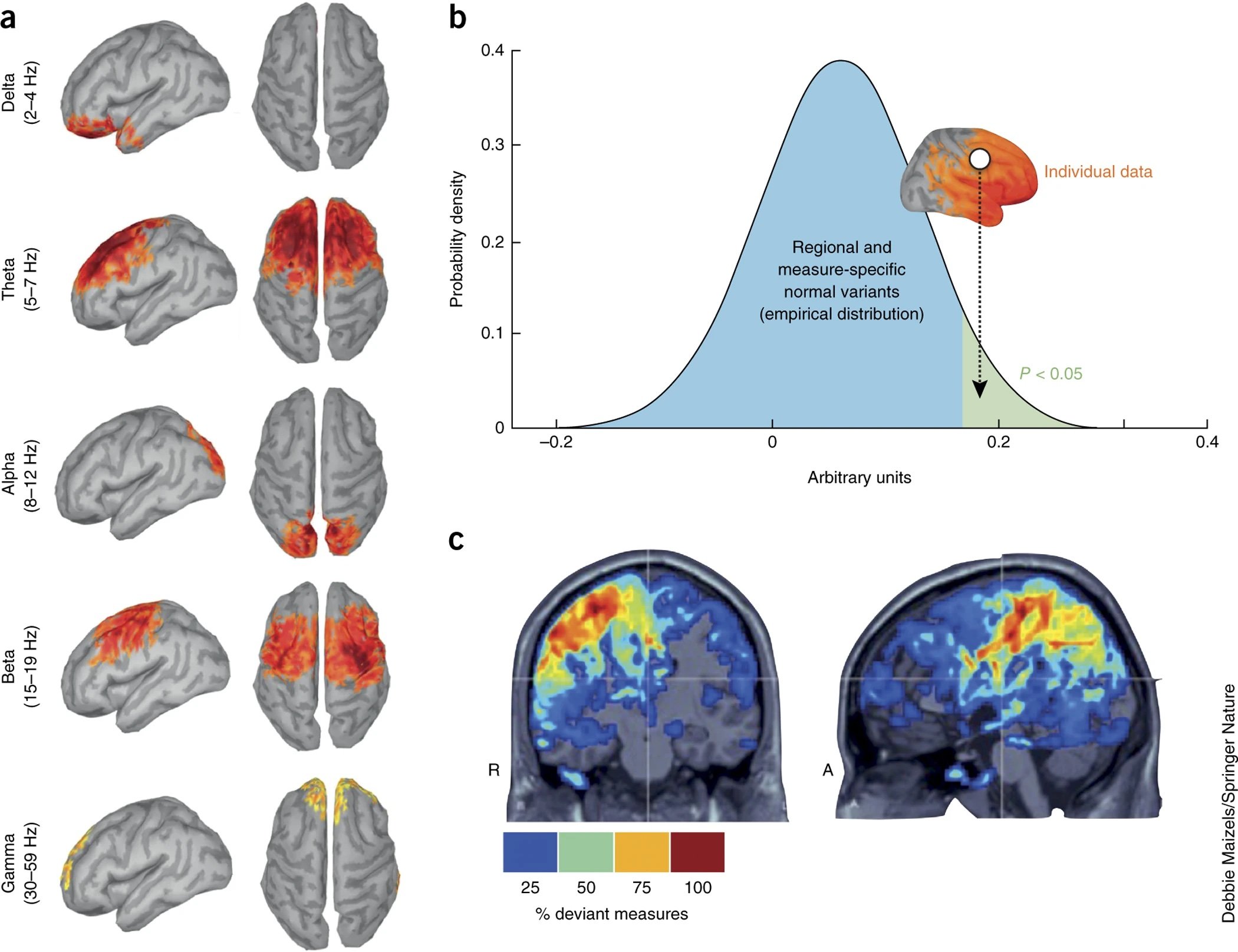
(a) Example of the outcome of an MEG imaging database. Ninety-six healthy participants were scanned in the resting state for 15 min with their eyes open. The average distribution of the magnitude of ongoing brain rhythms (from delta to gamma) found in the cohort are registered to and represented on a template cortical surface. Data were thresholded at 50% of maximum amplitude across the cortex. (b) Large data repositories such as OMEGA can be used to establish normative and patient variants of any analytic measure taken from MEG source signals. This is illustrated here, where for each measure and each brain location, the values obtained in a tested individual or group dataset can be assessed with respect to their empirical distribution in the databank. (c) Practical summarizing and visualization solutions can reveal the anatomical locations where, for example, a single or cumulated measures from the individual data from one patient deviate from those observed in the reference normative repository. Here, for instance, the colored brain locations indicate where abnormal strengths of oscillatory brain activity have been detected in the resting state and in multiple frequency bands in a patient with epilepsy. Figure adapted from this previous article.
To measure speech intelligibility, we used a new toolbox (audio-tokens; Figure 1A) developed by former lab graduate Peter Donhauser, after he moved with the group of Prof Denise Klein at McGill, that allows human listeners to interactively rate speech recordings from each patient. Both Peter and Denise are co-authors of this new study. We then tested how these speech impairments were related to changes in brain activity relative to healthy levels in the patients with Parkinson’s disease. In other words, we looked for unusual patterns in the brain activity of patients that were stronger in patients who had the greatest difficulties producing intelligible speech.
This figure describes the data workflow used in the present study to detect subtle alterations of neurophysiological brain activity in patients with Parkinson’s disease, compared to age-matched healthy controls.
We found that brain activity in a specific region of the cortex, the left inferior frontal gyrus, is related to speech intelligibility: speech impairments are more pronounced in patients whose brain activity in this region deviates the most from healthy levels. Decades of previous research have shown that this region is especially important for the cognitive aspects of speech production, rather than the mouth movements needed for articulating. This signaled that, at least in part, the speech impairments seen in patients are due to problems with speech planning.
We then examined the frequency-contents of the brain changes in this brain regions that were related to speech impairments in patients, which tells us how the relative speed of neurophysiological signaling is changing. We saw that these brain changes reveal an acceleration of brain activity in this region: patients with worse speech impairments have faster patterns of regional brain signaling than those who remained more intelligible.
Importantly, we also saw that speech impairments are also related to the amount of functional communication between the left inferior frontal gyrus and the other parts of the brain that control movement – patients with more “functional connectivity” between such regions produced more intelligible speech. This tells us that the integration of cognitive and motor processes across the brain is beneficial for speech production, and that the breakdown of this integration leads to worse speech impairments in Parkinson’s disease.
Together, these results give us new information regarding the brain-bases of speech impairments in Parkinson’s disease. Speech impairments that lead to worse intelligibility are attributable to problems with speech planning, as well as the integration of these plans with the movements required to produce them.
We hope that these new findings will inspire novel clinical interventions to ameliorate speech impairments in Parkinson’s disease through, for instance, brain stimulation – our results suggest that such therapies should aim to (1) decrease the speed of neurophysiological signaling in the left inferior frontal gyrus and (2) increase the connectivity between this regions and motor regions of the brain.
We have more studies of Parkinson’s disease motor and non-motor processes coming up. Please stay tuned and follow our work on social media for updates.